arsenic valence electrons|Iba pa : Tuguegarao Since the last shell of an arsenic ion has eight electrons, the valence electrons of arsenic ions(As 3-) are eight. What is the valency of arsenic? The ability of . Forcing A Sale Of Jointly Owned Property. The process is relatively straightforward: If the property is genuinely jointly owned (because in some cases it might not be); and; The only dispute is whether the property should be sold or not; then; All that’s required is a simple application to court.
arsenic valence electrons,Mar 23, 2023 Since the last shell of an arsenic ion has eight electrons, the valence electrons of arsenic ions(As 3-) are eight. What is the valency of arsenic? The ability of . There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h.
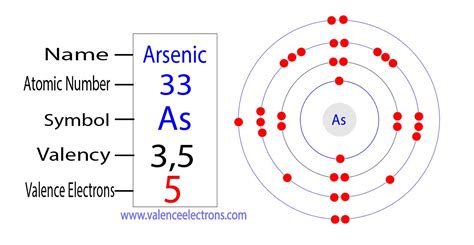
Arsenic has 5 valence electrons. Methods. We can write the valence electrons of arsenic using two different methods: #1 Using periodic table. #2 Using .
Arsenic ion(As 3-) electron configuration. The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. This electron configuration shows that the last shell of arsenic has .Arsenic is a chemical element of the periodic table with chemical symbol As and atomic number 33 with an atomic weight of 74.9216 u and is classed as metalloid and is part of .
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . Arsenic has 5 valence electrons because there are 5 electrons present in the outermost shell of the Arsenic (As) atom. Now let’s see how you can easily find the .
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .Iba pa Arsenic Electron Configuration: Chemical element, Arsenic has atomic number 33 and has the symbol “As”. Arsenic chemical occurs in many minerals like elements with a combination of .
Les électrons de valence de l’arsenic (As 3- ), en ont huit. La valence est une caractéristique numérique de la capacité des atomes d’un élément donné à se lier avec d’autres atomes. La valence de l’hydrogène est constante et égale à un. La valence de l’oxygène est également constante et égale à deux. Since the atomic number of arsenic is 33, the total electrons of arsenic are 33. Second, make a table of subshell and its maximum electrons. Calculate the maximum number of electrons .Elements. Finding the Number of Valence Electrons for an Element. Using the Periodic Table you can easily find the number of valence electrons for an element. These are the electrons in the highest energy level and the ones that are involved in chemical bonding. This is an essential skill in chemistry! Arsenic has atomic number 33. This means arsenic atoms have 33 protons and, if neutral, 33 electrons. The electron configuration for a ground state, neutral arsenic atom is: 1s22s22p63s23p63d104s24p3 or [Ar]3d104s24p3. So we can see that the electron configuration for arsenic has 5 electrons in the valence shell of the 4th energy level.Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of .

You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
The atomic number of arsenic is 33, which means it has 33 electrons. Now it is possible to find the orbital notation of arsenic very easily through electron configuration. That is, the orbital notation of arsenic is 1s 2 . Arsenic Valence Electrons Dot Diagram. Diagram or graphical form is the best way of studying the valence electrons. It breaks down the full-fledge electron configuration of a chemical element. We are having the Arsenic Lewis dot diagram here for the same purpose. This dot diagram will let you study the numbers of valence electrons .
arsenic valence electrons Iba pa sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . Arsenic is the 33rd element of the periodic table so its atomic number is 33. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, an . Figure 18.3.1 18.3. 1: (a) Arsenic and (b) antimony have a layered structure similar to that of (c) graphite, except that the layers are puckered rather than planar. (d) Elemental tellurium forms spiral .Comprehensive information for the element Arsenic - As is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Valence Electrons: 4s 2 p 3 Electron Dot Model. Chemical Properties of Arsenic. Electrochemical Equivalent: 0.93177g/amp-hr;

The electronic configuration of the stable form of arsenic, As(0), can be written as shown below to illustrate how the presence of five valence electrons allows arsenic to participate in chemical bonding, with an empty p orbital for electron occupation; this can also be illustrated by the electron dot model of arsenic (Figure 1–1). Let us calculate the number of Valence electrons in AsF 3. Arsenic Trifluoride comprises three Fluorine atoms and one Arsenic atom. Arsenic is in group 5 of the periodic table with the electronic configuration [Ar] 3d¹⁰4s²4p³. Therefore, the single Arsenic atom contributes 5 x 1 = 5 valence electrons. Fluorine is a halogenic compound. For this, the valency of sulfur is 4. Again, the electron configuration of sulfur excited state is S* (16) = 1s 2 2s 2 2p 6 3s 1 3p x1 3p y1 3p z1 3d xy1 3d yz1. In this case, sulfur has six unpaired electrons. Therefore, the valency of sulfur is 6.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Arsenic is [Ar] 3d10 4s2 4p3. A step-by-step description of how to write the electron configuration for Arsenic (As and the As 3- ion).In order to write the As electron configuration we .
arsenic valence electrons|Iba pa
PH0 · valence electrons for kids
PH1 · valence electrons chart
PH2 · periodic table valence electrons
PH3 · list of valence electrons for each element
PH4 · how to calculate valence electrons
PH5 · how many valence electrons arsenic
PH6 · arsenic valence electron configuration
PH7 · abbreviated electron configuration for arsenic
PH8 · Iba pa